
Physics: Matter, energy, waves, & particles

A classical explanation for the comlexity of all matter is that it is made up of simpler substances: Typically of earth, water, air, fire, and (later) aether.
Introduction
What makes up the universe?
Matter, energy, waves, and particles?
Humans first thoughts about matter (stuff, substance, objects) when they wondered what everything was made of. And then, wondered how small an individual piece of it (matter) was and how small it could be and still be the substance.
Ancient Greeks and Indians thought and wrote that all physical things (matter) was made from five basic elements, and organized them from bottom up: earth, water, air, fire and aether (matter in space).
The Greeks created the word, atom, meaning uncutable for the smallest particle that has all the properties of any substance that makes different kinds of matter in the universe. Today this could be atoms, elements, and molecules, depending on the substance.
Newton's laws describe how large matter interacts with gravity.
James Clerk Maxwell describes the laws of electromagnetism 1865
Werner Heisenbeg writes a complete mathematical framework for the theory of quantum mechanics. 1927. Certain quantities can never be known with precision. The more accurate one measures the a particle's speed, for example, the less certain we are of its position.
Erwin Schrodinger worked out the same only using equations that described waves: wave mechanics, 1935. The theory says that until a measurement is made the particle is in a superposition of states described by the wave function. The act of observation collapses th wave function so that the particle is seen to be in just one state.
Shrodinger's cat. There is a 50% change the dacay has taken place. Until the box is opened we can say the cat is in a superpositon of being both alive and dead. Strange.
Richard Feynman and Julian Schwinger, and Shin'chiro Tomonaga, 1948, develop quantum electrodynamics, a theory that explains how light and matter interact. It is based on Einstein's theory of special relativity and quantum mechanics. It is among the most precisely tested theory in physics.
Louis de Broglie, David Bohm (1952) develop pilot wave theory (de Broglie-Bohm theory) interprets quantum mechanics of paths particles follow, even when observed.
John Bell shows how entanglement could be tested with his Bell's theorem.
The standard model of particles is developed 1970. It describes how fundamental particles, quarks and leptons, interact with four physical forces.
Vocabulary
Particle is a source of matter that exists at a single point. It is the building block to explain mechanics (motion and the forces that creates it).
Quasiparticle is a quantum of energy in a crystal lattice or other system of bodies which has momentum and position and can in some respects be regarded as a particle.
Quantum Means count or quantity or discrete quantity.
- Can’t count or measure small quantities so use probabilities.
Quanta (plural quantum) is an individual and separate distinct discrete quantity of energy proportional in magnitude to the frequency of the radiation it represents.
Phonon is a quanta of energy or a quasiparticle associated with a compressional wave such as sound or a vibration that pass from atom to atom in a crystal lattice. They are boson excitations with different energy levels, and of any frequency level.
Energy is the ability to do work.
Work is moving something against a force.
Defined as:
W(ork) = f(orce) * d(istance) * cosine θ (the angle of force different than the direction applied.
If the direction of force is the same as direction of motion, then θ is 0 and cosine of zero is 1)
Together, these are the building blocks of all kinds of matter.
Matter contains a huge amount of energy:
Einstein showed with, E = mc2
Energy can travel as electromagnetic waves: heat, light, radio, x-ray, microwave, gamma ray.
Your body metabolizes energy as chemical changes.
Larry M. Silverberg & Jeffrey Eischen explain matter as being made of energy fragments. Source
Conservation of energy
Nature, back to nature, balance of nature, harmony of nature, Unlimited abundance
Free energy technologies may be $ free, cheep, or inexpensive, However, energy is always conserved so not physics free. For example a refrigerator using power connected to the grid will cost money for the electricity. The same frig could be powered off the grid with solar panels, not cost money beyond the cost of the equipment. Or a Zero pot cooler could chill fruit and veggies without electricity. However, all three will transfer the same amount of heat energy if each are to maintain the fruit and veggies at the same temperature for the same length of time.
Superposition is when objects exist in two or more states at the same time.
Entangled is when objects are exactly the same and in different positions and behave similarly. Example is like if two people were each tossing a coin and the results are that everytime one comes up tails the other comes up tails and whenever one comes up heads the other comes up heads. That's quantum entaglement.
Entanglement does not mean that information can be know about the state of the sytem in two locations instantaneously. Information can not travel faster than the speed of light. Only the interna state of the system can be affected instantaneously. The coupled dynamics of a quantum mechanical system can be independent of the particles' individual locations. Much like the tone of a piano does not tell you much about the color or size of the piano.
What happens to the internal state of one particle can be experienced by instantaneously by the internal state of another particle, if they are entangled. Regardless of where they are no matter how far apart they are. Weird.
Wave is used to explain electromagnetism, electric and magnetic fields and their interactions. They exist everywhere except at the point that creates them.
Duality - wave or particle or both
Field used to explain actions across space or interactions at a distance.
Constant is a value that occurres continuously over a period of time:
Universal constants (same anywhere in universe)
Examples of constants
- Speed of light or how fast light travels
- Newton's Gravitational constant or the force of gravity between two objects in space
- Plank's constant or the amount of energy exchanged between atomic or molecular systems. It is the relationship between the energy of a photon and its frequency, the mass-energy equivalence, and the relationship between mass and frequency. specifically one photon's energy is equal to its frequency multiplied by the Planck constant. The constant is symbolically denoted by h.

Micro matter
Atoms
Over time atoms were found to be composed of hadrons: protons, neutrons,(with an atomic mass of about one), and electrons. With the protons and neutrons located in the nucleus (together called nucleons) with most nuclei surrounded by a swarm of electrons. Altogether they make an atom, with a diameter of about 10-8 cm.
The total average number of protons and neutrons is the atomic mass, with each element having different mass numbers, based on the number of protons. Since the number of protons determines the kind of element, the atomic number is unique for each each element. Also since the average electrical charge of an element is neutral, the atomic number also represents an equal number of electrons.
However, not all nucleons (nuclei) of an element have the same number of neutrons. As the number of protons increase for different elements, the number of neutrons increases a few more than the number of protons increase. This results in atoms, with the same number of protons, sometimes having slightly different numbers of neutrons, which make different atomic masses for different varieties of the same elements (isotopes). For example: fluorine has one isotope and tin has ten with most having at least two.
This creates two values: the atomic number, which is always a whole number representing the number of protons in an element and atomic mass, which ranges from slightly more than double the atomic number to slightly more than 2.5 times, because of the different amount of neutrons. The atomic mass, or weight, is calculated from the average of large amounts of each element as it is found in nature. Which will include its isotopes (elements with different numbers neutrons). Because of the different isotopes, the atomic mass (number of protons & average number of neutrons) is slightly more than twice the atomic number (the number of protons).
Matter vibrates
Resources about Elementary Particles
- A good source
- Article - Probing the frontiers of particle physics
- The Particle Adventure - Electronic book.
- Sample graph for motion of a particle.
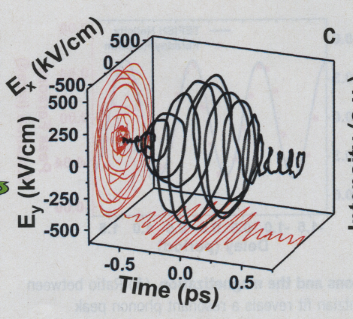
Source Science November 10, 20323
Elementary particles

Bosons
Bosons include
- Photons - light particle waves
- Gluons - hold quarks together in neutrons and protons
- Gravitons - exist?
Bosons can exist in the same state as the same time. and they tend to clump together. Like lasers with streams of photons with the same quantum state.
Photons
Photon is a particle that represents a quantum of light or other electromagnetic radiation. A photon has energy proportional to the radiation frequency but has zero rest mass.
Photons are so sensitive they are destroyed when measure. Vertical polarized light can pass through vertical slit. Horizontally polarized light cannot pass through vertical slit, but bounces off.
Fermions
Fermion is a particle that follows Fermi–Dirac statistics and obey the Pauli exclusion principle. They have half-integer spin (1/2, 3/2, ... ). Electron, proton, leptons, quarks, ... There are 12 types of elementary fermions - 6 quarks and 6 leptons.
Fermions keep to themselves. No two can exist in the same quantum state at the same time. They make solid mateer
Quarks
Quarks - have color symmetry and a spin of 1/2. The protons and neutrons in the nucleus of an atom are made of quarks. There are six types or "flavors" of quarks:
- up-type quarks (up, charm, top)
- down-type quarks (down, strange, bottom)
- Each comes in three colors - charges: red, green, and blue.
Leptons. (spin 1/2)
Electrons
Electrons and its two super massive sisters - muons and taus are normally found around an atom's nucleus.
- Electrons are electrically charged and drive life.
- Electrons cascade up and down.
- When they cascade down they give off energy.
- Sometimes when an electron cascades down it can pull up an electron (flavin).
- Electrons interact with matter about 10 000 times stronger than x-rays.
- Spin filtering suggests that a chiral medium can only allow electrons with a specific spin orientation to pass through while those with spin orientation not aligned with the chiral configuration would get blocked, leading to an equilibrium spin current across the chiral medium.
- By contrast, the spin polarization concept suggests that electrons passing through a chiral medium experience an effective magnetic field that orients the unpolarized carriers, allowing all carriers to be spin-polarized electrons.
Muon - 200 electron masses. Muon g-2 (muon gee minus two) experiment found each muon is charged (like a little bar magnet) and will circle a magnetic field. If a muon enters a magnetic field in a perpendicular orientation it will precess like a compass needle. Theory suggests if a muon is polarized in the direction it travels it locks their will in orbit. However, the muon continually emits and reabsorbs other particles (pop into and out of existence) which increases the muon’s magnetism and will precess faster than it circulates. It can emit and reabsorb any particle and the change in magnetism can signal the particle. Positively charged muons decay into positrons spit out in the direction of the muon’s polarization. Therefore, muons can be tracked by detecting positrons.
Positron - a positive particle like an ordinary electron in mass but with opposite charge. Antimatter. Nice article about antimater and its Discovery by Paul Dirac of a positron as antimater of an electron.
Neutron
Neutron - will decompose into a proton + electron + neutrino
Neutrinos, the electron neutrino, muon neutrino, and tau neutrino. Lightweight and weakly interacting.
Proton
Protons are made with three quarks and held together with gluons. They have a positive charged and a radius = 10 -15m
Composite particles and more
Composite particles - hadrons - composed of other particles. Hadrons are made of two or more quarks and held together with the strong force. Therefore, are not fundamental. They include baryons and mesons.
Neutron - will decompose into a proton + electron + neutrino
Protons are made with three quarks and held together with gluons. They have a positive charged and a radius = 10 -15m
Beta decay - two types when a nucleus has either too many protons or neutrons
- + beta decay when a proton in a parent nucleus decays into a neutron that remains in the daughter nucleus, and the nucleus emits a neutrino and a positron (a positive particle like an ordinary electron in mass but with opposite charge).
- - beta decay a neutron decomposes to a proton + electron + neutrino
Baryons. (spin 1/2, 3/2) Baryons are fermions composed of three quarks. The most important baryons are the two nucleons: the proton (up-up-down quarks) and the neutron (up-down-down quarks). Some other baryons are the sigma, lambda, xi, delta, and omega-minus.
Mesons. (spin 0, 1) Mesons are bosons composed of a quark and antiquark. Some mesons are the pion, kaon, eta, rho, omega, and phi.
Antiparticles. All particles have a corresponding anti-particle that is identical in many ways but opposite in others; for example, the mass and spin are the same but the charge is opposite. An uncharged particle may be its own anti-particle.
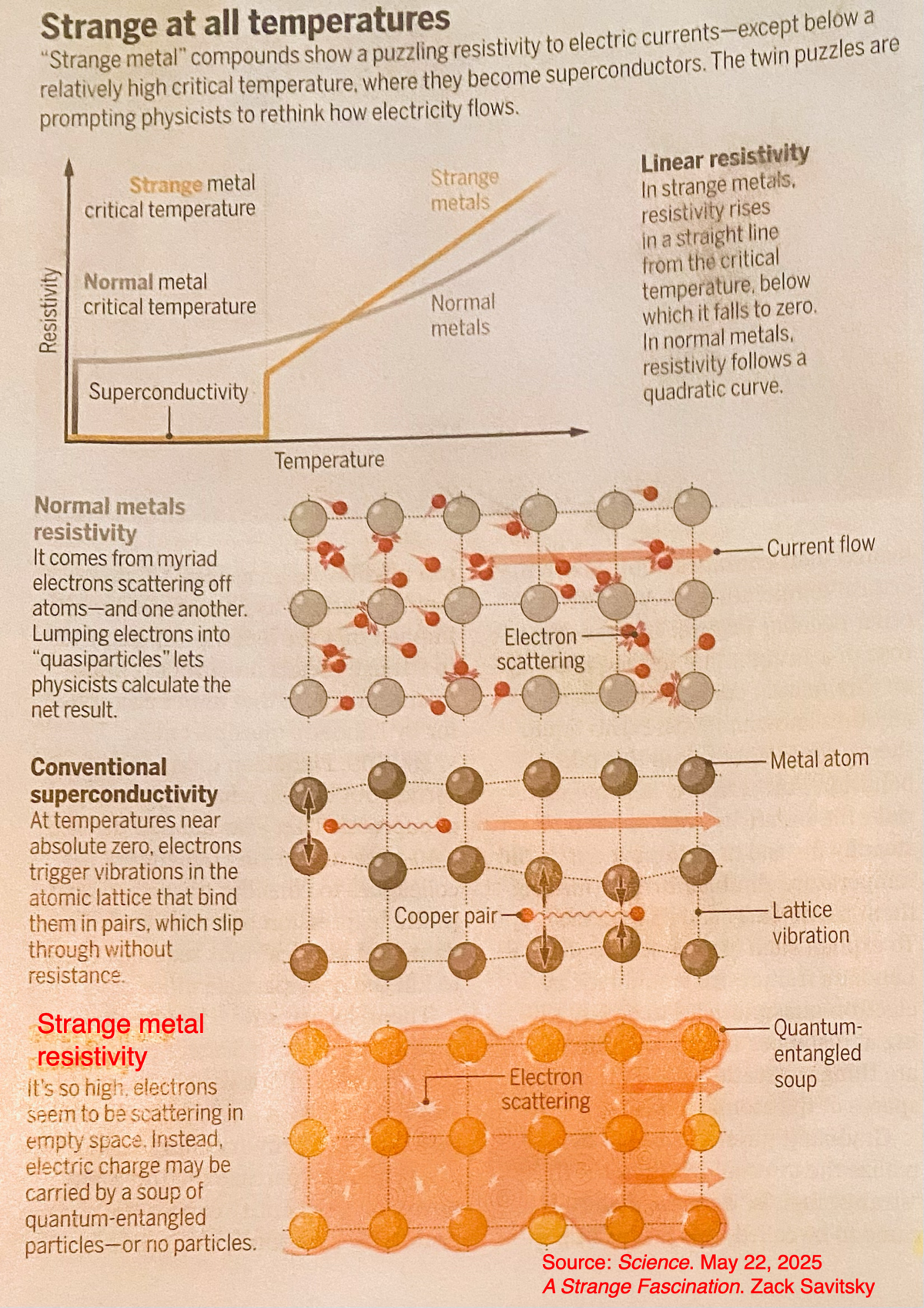
Dark energy as matter (normal matter [which gravity pulls normal matter into galaxies and holds them together] and unseen cold dark matter, [CDM] which gives all of space a springiness puching against gravity) grows with space and creates a mysterious force that is accelerating the expansion of the universe. Some observations suggest this force may have varied over time. This dark energy is represented as lambda and known as the cosmological constant in Einstein's theory of gravity, general relativity. Dark energy is described by the parameter w, the ratio of pressure over its enrgy density. Lamda-CDM gives w a precise and constant value of -1. But some believe it may have changed from -1.4 in the distant past to -.8 today. Problamatic as some believe it can't go below -1.
Bose Einstein condensate - single quantum wave 1924.
Super partner = super symmetry = link between bosons and fermions that make up matter. Hypothetical partners - Every standard particle may have a superpartner particle: a fermion for each boson and a boson for each fermion
Elements - Noble gases (monatomic) He, N, Ar, Kr, Xe, Ra
Quasi-particles and other non-particles
Many quantized states are not real particles, but are conveniently named and treated as if they were real particles. Some are the quantized modes of collections of particles.
- Soliton. A stable solitary wave packet arising from a combination of waves. Solitons are found in many physical phenomena, large and small.
- Phonon. A quantized sound wave.
- Electron hole. The absence of a negatively-charged electron in a semiconductor, treated as if it were a positively-charged particle.
- Cooper pair. A pair of electrons (fermions) with opposite spins making the net spin zero in a superconductor, treated like a single boson. Most common are s-wave superconductors with spatially symmetric wave functions.
- Exciton. A bound state of an electron and an electron hole. Excitons, are quasiparticles of mobile energy concentrations in crystals formed by excited negatively charged electrons bound to a positively charged hole, are ubiquitous in semiconductors and optoelectronic devices. Adding a charge carrier to an exciton creates additional charge and spin degrees of freedom, converting it into a fermion with increased effective mass. Three fermions form a trion, often requiring optical excitation. However, these quasiparticles are short-lived, with a binding energy of about 1 Meg.
- Magnon. A quantized spin wave.
- Plasmon. A quantized plasma oscillation.
- Polaron. A quantized polarization field.
Standard model (SM) of particle physics
Standard model (SM) of particle physics is based on the idea that each particle is an excited state of a corresponding field and the force between them.
A force field arises when a third particle is exchanged. The SM fails to explain some observations: dark matter, dark energy, how atoms survived the Big Bang, value of the cosmological constant (lamda = 2.036 * 10 -35 s-2), ...
Cosmological constant was created by Einstein when he thought the Universe was not expanding.
Matter in the universe
The standard model (SM) does not account for our present observations of the amount of matter in the universe.
Source Probing Fundamental Particles with Molecule. Science July 7, 2023
Matter and antimatter particles -
Matter - antimatter asymmetry requires forces that change strength when matter and antimatter particles are interchanged (an operation called conjugation C) with their mirror image particle (called parity transformation P) known as conjugation parity (CP). These forces violate CP symmetry in the standard model (SM) model. To preserve total symmetry CP is said to violate time-reversal (T) symmetry so that CPT is preserved.
The electron electric dipole moment (EDM) is an intrinsic property of an electron such that the potential energy is linearly related to the strength of the electric field. An electron's EDM must be collinear with the direction of the electron's magnetic moment (spin).
Within the SM of elementary particle physics, such a dipole is predicted to be non-zero but very small, at most 10−38 e·cm, where e stands for the elementary charge. The existence of a non-zero electron electric dipole moment would imply a violation of both P (parity) and T (time reversal).
In the SM, the electron EDM arises from the CP violation components of the CKM matrix. The moment is very small because the CP violation involves quarks, not electrons directly, so it can only arise by quantum processes where virtual quarks are created, interact with the electron, and then are annihilated.
Magnetic dipole is the closed circulation of an electric current.
An electric dipole is a separation of positive and negative charges.
Chirality and handedness
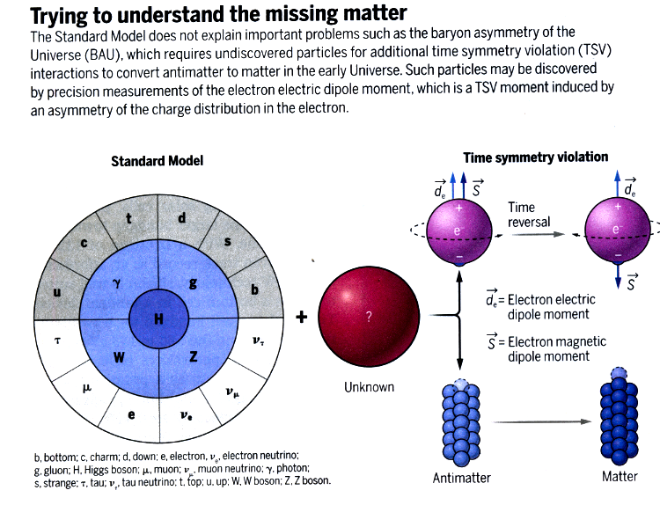
A chiral phenomenon is not identical to its mirror image (asymmetric). Hands - left and right are chiral because they are mirror images of each other, but however you reorient them, you will not be able to make them overlap.
- The spin of a particle may be used to define its handedness or helicity.
- Spin is the intrinsic spatial orientation of quantum particles
- Massless particles have the same spin and chirality.
- A symmetry transformation between the two is called parity.
- Parity refers to whether the properties of the particle remain the same when some of its spatial coordinates are flipped, like comparing the particle with a hypothetical mirror image
- Invariance under parity according to Fermi–Dirac statistics is called chiral symmetry.
- Chien-Shiung Wu in 1957 demonstrated that parity is not a symmetry of the universe.
Chirality in nature
Chirality-the inability of an object to be superposed onto its mirror image is important to biological, chemical, and physical processes.
For example, amino acids and sugars found in all living organisms are chiral, bearing a specific handedness (left or right) that affects the reactivity of the molecule. Crystals can also have inherent chirality and exist as a pair of two distinct states that are mirror images of one another (enantiomers). Chirality of crystals is intimately connected to electromagnetic properties and dictates how light interacts with a material. However, modifying chirality in solids is challenging because it requires structural rearrangement.
Two different forms of chirality exist: geometric and motion-based. A helical object, such as a screw, has geometric chirality in which the object cannot be super-posed onto itself after a rotation followed by a reflection (no rotoreflection symmetry). By contrast, motion-based chirality can exist in systems that have an achiral geometry (its mirror image is identical to the original).

Vibrations and waves
Vibration is an oscillation (a back and forth motion) like a vibrating clarinet reed, drum head, and sloshing liquid in a container. An oscillation is local event. A wave travels.
Vibration result from the interaction of moving particles. For example vibrations of salt or water on a table will, from a distance look like waves, but close up look like random jumping particles.
Vibrations depends on the space between particles, force, position of a force relative to the particles around it, the density of particles, volume of space around the particles, rate of motion (temperature - cool - slow, hot - fast burn). See video of particles of four different colors of sand on a table vibrating and interacting.
Waves transfers energy from one place to another without a net flow of mass. When motion is viewed as waves, particles appear to travel in a pulse or in ripples. Motion that can be standing or traveling and in the direction the particles are moving (longitudinal) or at right angles (transverse) or a combination of both, water waves. . While waves are sometimes thought of as a collection of particles, it is an over simplification in some ways.
First, water/smoke, ... waves are not actually one wave after another. Each wave is composed of many particles moving and interacting. Therefore, what we see is a collection of particles in motion interacting with each other particles. The wave is an explanation of the collective action of the particles. Which can be overlooked when explanations use the invention of the words, wave or waves.
Second, the interaction of water particles and other substances that are floating, suspended, or sunk is explained with Newtonian physics, which works on particles (10-4) or larger scale. However, photons, electrons, and all other subatomic particles are best explained with Einsteinian physics, which works on particles (10-5) or smaller scale.
Properties of waves include:
- Crest is the high points of a wave.
- Trough is the low point of a wave.
- Wavelength is the distance between two successive wave crests (high points) or troughs (low points) or other similar paired points.
- Amplitude is the maximum distance of a particles vibration. It is measured in different ways. From equilibrium to top of crest, from high crest to trough, and average height of vibration from rest (its undisturbed position ).
- Period (t) is the time required for one vibration cycle
- Frequency (f = 1/t) is number of cycles in a certain time, usually 1 second.
- Speed (v = frequency x wavelength) is the frequency times the wavelength or the number of vibrations times the distance traveled with each vibration.
- Oscillate means to move back and forth at a regular speed like a pendulum. Waves do not oscillate, unless thy are reflected, like an echo or reflected with a mirror.
Waves and energy
What we see as light and heat are reproduced, transferred, and changed with interactions of energy. Energy that leaves the sun and is reproduced when it reaches Earth and interacts with Earth particles on Earth or in the atmosphere.
Solar particles, that produce heat and light, generally do not leave the sun. Energy in forms of radiation, waves, spirals, .. travel to and interact with Earth.
Lens compress and focus energy they don’t multiply energy.
Fields
Fields are everywhere and act like liquids as ripples and waves. When a wave is formed a particle is created.
Electromagnetic force as quantum fields - quantum electrodynamics (QED). Ripples in one field can create ripples in another field.
Quantum electrodynamics (QED) describes the forces between electrons and matter and light and electrons. Proton electron mass ratio. Mp/Me.
Forces, fields & matter
Four fundamental forces and their fields. Elementary bosons - follow the Bose–Einstein statistics - spins are integers. They transmit forces that function as glue, may stick together, can occupy the same quantum state.
- Strong nuclear force gluon fields is on a smaller scale (< less than 0.8 fm, radius of a nucleon) is the force carried by gluons that holds quarks together to form protons, neutrons, and other hadron particles. Gluon has spin 1 the nuclear force or color force.
Source shows nice animation of particles - Weak nuclear force W± and Z fields cause radioactive decay. W± and Z bosons carry the weak force and have spin 1.
- Gravity warps space time, force between masses, gravity waves, ripple space time. Missing from the SM. Graviton has spin 2. Gravitons [predicted] carry the gravity force.
- Electromagnetism photon field carry the electric and magnetic fields. Photon has spin 1 and are particles of light. Light is an electromagnetic wave. Photons - can boost electron levels up and down energy. W1, W2, W3, and B bosons carry the electroweak force and have spin 1. When the electroweak force split into the electromagnetic and weak forces, the W1, W2, W3, B, and Higgs remix to make W±, Z, photon, and Higgs.
- Altermagnetism - is a material in which the atoms and their spins rotate independently, giving them the properties of both ferromagnets and antiferromagnets.

* Higgs boson. (spin 0) The Higgs boson is an excitation of the Higg's field. The Higg's field gives particles of matter and the W & Z bosons their inertial mass, but not massless particles like the photon.
The Higgs boson mass of 125... gigaelectronvolts (GeV) is identified by its two quantum-mechanical properties of quantum spin (Spin is the intrinsic spatial orientation of quantum particles) and parity (parity refers to whether the properties of the particle remain the same when some of its spatial coordinates are flipped, like comparing the particle with a hypothetical mirror image), which support a Standard-Model interpretation.
The Higgs boson, like most kinds of particles in nature can never directly be seen, it is unstable and immediately after being produced transforms into lighter particles through a process known as particle decay.
In the Standard Model, the Higgs boson has no spin (“0”) and “even” parity. At the time of the discovery, the fact that the Higgs boson transformed into photons meant that – unlike all other elementary bosons we know – its spin could not be 1: photons have a quantum spin of 1 themselves, so a particle transforming into two photons would have a spin of 0 (with the two spins of the photon cancelling out) or 2 (if the two spins add up).
However, large numbers of observations of Higgs bosons have nor provided enough information to know if what was observed is the Higgs boson predicted by the Standard Model.
Today, (2024) we know with great precision what its mass is, what its most abundant transformation channels are and how it is produced in the first place. But a lot remains unknown, about both the Higgs boson and the quantum world in general.
Time
If electrons traveled at light speed, they would think they arrived at their destination when they left their point of origin. (Like photons of light.)
Molecules
Molecules are held together with chemical bonds
Chemical bond is a fairly stable attraction caused by an electrical force that holds atoms, ions, and molecules together, which makes a chemical compound.
Covalent bond or ionic bond.
Covalent bond is a chemical bond made when atoms share one or more electrons. Often each atom shares an electron to make a pair of shared electrons.
Ionic bond is a chemical bond between ions with opposite electrostatic charges where one atom gives up one or more electrons to another atom and holds them together by that force of attraction. Unequal sharing. Clump like magnets in crystal structures.
Examples of ionic bonded chemicals or molecules
- Table salt - NaCl sodium chloride, Kl potassium iodide is added to salt to make iodized salt for thyroid health
- Fluoride toothpaste - NaF sodium fluoride
- Baking soda NaHCO3
- Antacids include different kinds of ionic compounds
Hydrogen
The simplest molecule has two protons bound by an electron.
A Hydrogen ion coded as ( ≡ means identical to)
H2 ≡ p+ + p+ + e-
A hydrogen deuteride ion is a hydrogen molecule, with one proton replaced by a deuteron.
HD+ ≡ p+ + d+ + e-