Physical Science Activities - Heat Energy, energy transfer, temperature observation, evidence, variables, measurement, and reasoning to understand. (3rd - 5th Grade)
Contents
- Science content focus - what science says - enduring understanding, big ideas, generalizations
- Science inquiry focus - how we use science to collect information to understand
- Activity sequence
- Resources and materials
- Pedagogical ideas
- Lessons and activities detail
- Assessment
- Lab notes
Overview
This investigation explores why different objects feel warmer or colder to the touch. Starts with a collection of different objects that are arranged by how warm they feel. Activities continue to help develop the idea that heat energy is transferred by touching.
Physical Science - Heat Energy, energy transfer, temperature
(what science says - enduring understanding, big ideas, generalizations)
Objects feel warmer or colder to the touch. We refer to that sensation as temperature. The amount of heat energy in an object determines its temperature. Heat energy will transfer from warmer objects to colder objects.
Related concepts and facts -
- Energy is a property of matter that is related to doing something - heat, chemical change, motion, light, magnetism, nuclear change, electrical.
- Heat can be produced in many ways, such as burning, rubbing, or mixing one substance with another.
- Heat is transferred from a source to a receiver.
- Heat is transferred in predictable ways, flowing from warmer objects to cooler ones, until both reach the same temperature.
- Heat can be transferred during a chemical, electrical, magnetic, light, mechanical, or nuclear reaction.
- Objects that give off light usually give off heat.
- Mechanical energy is usually related to heat through friction.
- Hot and cold objects will transfer heat energy until they reach equilibrium.
- Some materials conduct energy better than others.
- Some materials can transfer heat by contact or at a distance
- Heat is almost always a result of energy transfer.
- Heat can be transferred by touching of particles (collisions of atoms conduction), or through space (by rays radiation) or currents in a fluid (convection).
- Heat energy is the disorderly motion of molecules and in radiation.
- Insulator is a material that has a slower transfer of energy.
- Conductor is a material that easily transfers energy.
Outcome
Heat energy is related to the amount of particle (atom, molecule) movement in a substance.
Specific outcomes -
- Describe energy as ...
- Describes hot and cold as a measure of heat energy.
- Describes temperature increases and decreases as directly related to the amount of heat energy.
- Describes heat energy as directly related to the amount of random motion of the particles (atoms,molecules).
- Describes the transfer of heat energy as a change in the random motion of the particles (atoms, molecules) caused by the touching of objects with different amounts of heat energy.
- Describes the exchange of heat energy as a product of the temperature differences and mass differences of all the objects involved in the exchange.
- Use observation to compare how different surfaces vary: smooth, rough,bumpy, air pockets... can affect the transfer of heat energy.
- Identify surfaces as a variable that changes from object to object.
- Observe the different surfaces and see if there is a clue on how each might relate to how the energy is being transferred.
- Describe the relationship between the kinds of surfaces and the transfer of heat energy.
- Describe and explain that the flow of heat energy between different surfaces is different depending on the surface of the objects.
- Describe how heat transfer affects our daily lives and relates to the world in which we live.
Inquiry - processes - variables
(How science inquires - process, skill, methodolgy)
Variables are conditions that can be changed and that can affect outcomes.
Related concepts and facts -
- Variables include, size, shape, temperature, amount, volume, rate, ...
Outcome
Identify variables and describe how they operate to effect other variables. (operational definition).
Specific outcomes -
- Identify variables and suggest how they change.
- Identify different variables and describe how they are conditions that can be changed and that can affect outcomes.
- Compare variables. (different surfaces with different shapes and properties, made from different materials that have different properties ...
- Combine variables to see if there are patterns or relationships to use as explanations (variables that affect energy transfer).
- Identify variables and describe how they operate to effect other variables. (operational definition).
Inquiry - processes - measurement
(How science inquires - process, skill, methodolgy)
Temperature is the measure of heat energy.
Related concepts and facts -
- measurement is a way to make more accurate or better observations
- thermometer is a tool that measures the temperature (heat energy)
- water freezes at 0 degree and boils 100 degrees Celsius
- water freezes at 32 degree and boils 212 degrees Farenheit
Outcome - measurement
Identify variables and describe how they operate to effect other variables. (operational definition).
Specific outcomes -
- States that a thermometer is used to measure temperature.
- Knows the approximate relative temperatures of freezing, room temperature, summer day, and boiling.
- Accurately and safely uses thermometers with different scales to decide an appropriate range or error and measure temperatures from below freezing to boiling.
- Knows how to safely and accurately take appropriate temperature readings and understands that temperature scales are dependent on arbitrary relative references (boiling or freezing) and the degradations one chooses to use between them.
Activities - to provide sufficient opportunities for students to attain the targeted outcomes.
Possible Activity Sequence
- Order objects by how cold or warm they feel.
- Discussion
- Take the temperature of the objects
- Discussion
- Temperature and perception
- Look at objects with microscope
- Create a model for possible explanation that explains the observations - both our touching sensations and the observations of the thermometer readings.
![]()
Pedagogical ideas
Focus questions - What is temperature? What makes objects feel warm and cold? Why do some things feel cold and others feel warm? How do we determine? How can we make better observations? How do the observations we make provide more accurate information? Could we think of an experiment that we might do to expand our theory of surface shape effecting the transfer of energy?
Scoring guides
![]()
Activities
Exploration
Activity: Order objects from hot to cold by touching them
Materials: 6-8 objects with a range of different surfaces that conduct heat energy from poor to real good: cotton ball, metal, plastic, cloth, chalk, and wood block or pencil; thermometer

Activities
What is the coolest and warmest of the objects? (Exploration)
Have the collection of objects displayed in the classroom so students can see that they have been there for hours or minutes as appropriate for their coming and going to class. Pass one collection of the similar objects to each group and ask them to arrange the objects from the coolest feeling to the warmest feeling and record the results in their lab notes. Students should become disequilibrated.
- Have students organize the objects from hot to cold by touching them
- Discuss with the group, what in their ordering is similar and different (Did everyone put the metal cube as the coldest? Did everyone put the any other as the warmest?).
- Ask students why they feel different.
Activity: Temperature and perception
Materials: Three cups, hot water, cold water, and medium water.
Activity procedure: Place the index finger of your right hand into hot water and the index finger on your left hand into cold water, count to 30, and put both fingers into the medium cup.
Invention
Activity: Actual temperature of the objects
Materials: Thermometer
Activity procedure: Take the temperature of each object and write it in your lab notes.
Activity - Look at objects with microscope.
|metal cube | candle | chalk | wood oak block | plastic cube | fabric | styrofoam | cotton ball |
Activity: Energy transfer model
Focus question - What causes your finger to feel a temperature difference?
Make a model of the interactions that explain how the temperatures can actually be identical and feel differently.
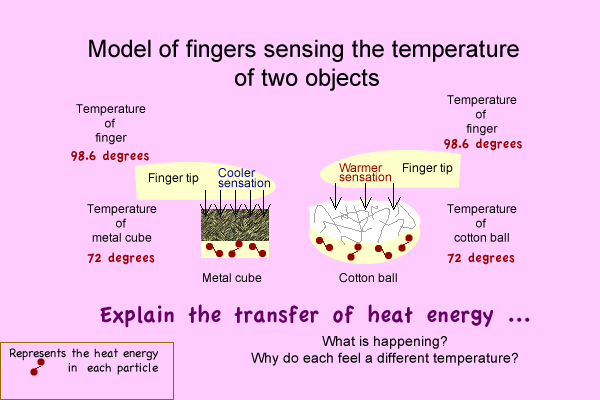
Write an explanation.
![]()
Lab Notes
Select six different kinds of materials that are not near a heat source, outside window, or in the sunshine. Write a general description to identify each in a chart. Imagine that you touched each material and predict how warm or cool it would feel if you touched it. Then actually touch a sample of each and write your observation of how cool or warm that it actually felt.
| Material | Prediction | Observation | Comments |
| 1 | |||
| 2 | |||
| 3 | |||
| 4 | |||
| 5 | |||
| 6 |
- Which type of material felt the warmest?
- Which type of material felt the coldest?
- Order the materials from warmest to coldest?
Warmest
1.
2.
3.
4.
5.
6.
ColdestWhy do think the materials felt different?
A person made the following statement: "The different materials do not have different temperatures. Each of the materials is about as warm as all the others even though they felt different." Why do you think she/he made this statement?
- What evidence could you collect to come to this conclusion?
Hold a thermometer for about one minute on each of the materials and find all of the temperatures.
- Does the differences match the differences felt?
- What is the air temperature in the room at their original locations?
- What does the data suggest?
Put all the materials in the same place for a period of time. Do you think they will have the same temperature?
- What do you observe?
- What do you conclude from this additional information?
Is it possible that the difference in the temperature felt is caused by some other property, beside temperature?
- Another property of the materials?
- What property do you suspect might be involved?
- Could different materials transfer heat at different rates?
- How do the differences in the temperature relate to the kind of material each is made of?
- For example, does plastic transfer heat less than metal?
- If the plastic would transfer less heat from your hand, then would that cause it to feel warmer than the metal or colder?
- Would it feel colder the more heat transferred from your hand or warmer?
- Would if feel hotter the more heat transferred from your hand or colder?
- Could the rate of transfer be related to the properties of the different materials rather than the temperature of the different objects?
- How does the transfer of heat from you hand makes them feel different?
Create a model to explain why the observations - both our touching sensations and the observations of the thermometer readings.
Model
Extensions
How would you answer the following question posed by a student? "On a cold day why do the handlebars of my bike feel colder than the handle grips?"
What kinds of thinking processes did we use to solve the problems.
What processes would scientist say that we used?
| Thinking process | Scientific Process | How did it help or how did we use it? |
What feedback from your actions was especially helpful?
Dr. Robert Sweetland's Notes ©







